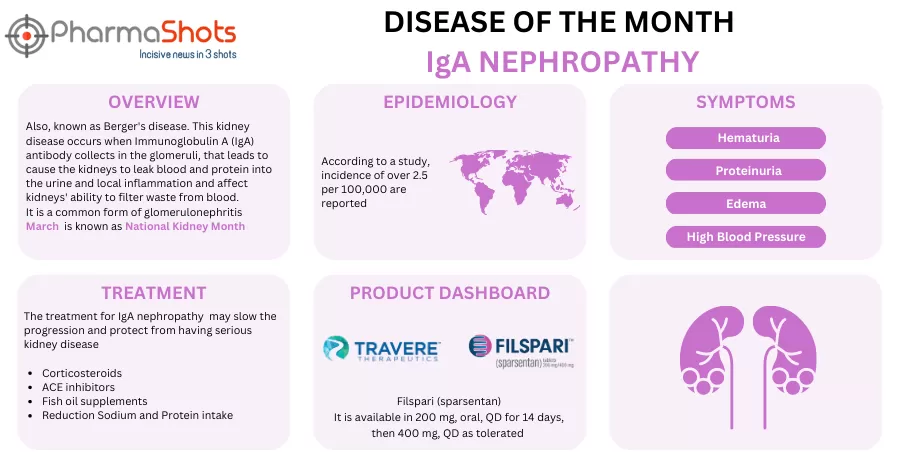
Travere Therapeutics Reports sNDA Submission of Filspari (sparsentan) to the US FDA for Treating IgA Nephropathy (IgAN)
Shots:
- The sNDA was based on 2-yr. confirmatory data of the P-III (PROTECT) trial assessing the safety & efficacy of Filspari (400mg) vs irbesartan (300mg) for treating IgAN patients (n=404, 18yrs. & above) with persistent proteinuria post at least 50% of max. label dose & maximally tolerated ACE or ARB therapy. It is intended to convert accelerated approval, granted in Feb 2023, into full approval
- The data demonstrated long-term preservation of kidney function, proteinuria reduction & a meaningful difference in eGFR slope with Filspari vs irbesartan
- Additionally, CSL Vifor (its EU commercial partner) has received CHMP’s recommendation for CMA to treat IgAN. Decisions of both the US FDA & the EC are expected in Q2’24
Ref: Travere Therapeutics | Image: Travere Therapeutics
Related Posts:- Travere Therapeutics’ Filspari (sparsentan) Receives the US FDA’s Approval for the Reduction of Proteinuria in IgA Nephropathy
PharmaShots! Your go-to media platform for customized news ranging for multiple indications. For more information connect with us at connect@pharmashots.com
Click here to read the full press release
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.